-40%
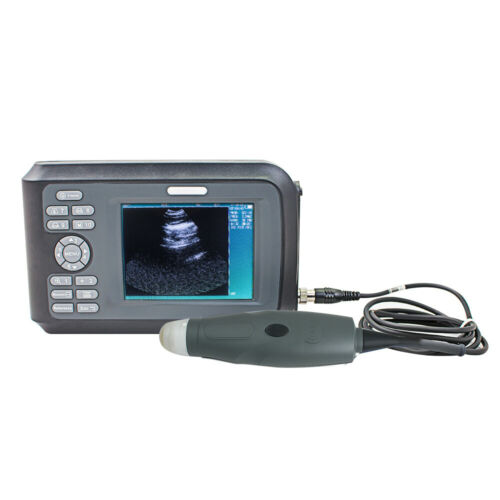
Full Digital Laptop Ultrasound Scanner Convex & Linear 2Probe with 3D software
$ 849.55
- Description
- Size Guide
Description
Laptop/Portable Ultrasound Scanner Machine Convex Linear 2Probes/Transducer FDAADescription:
Tips:
For USA Buyer: ship from USA warehouse . Fast delivery (2-5 days);
For Other Buyer : ship from China Warehouse.7-10 days delivery.
Features For Ultrasound Scanner:
High-frequency beam-former to ensure best resolution of the image
8steps for TGC and digital over-all Gain grasp the high-quality image
Whole-course real-time continuous dynamic focusing and aperture obtains high-resolution and picture in picture in
Super professional software packages to have the scanner widely used in Obstetrics Gynecoloyg,Urology, Cardiology ,Small parts.
Easy for operators with the comfortable application and professional software package.
It is the best choice for clinical diagnosis,because it makes clinic diagnostic more convenient and effective.
Specification For Ultrasound Scanner:
Screen Size
10.4 inch
Display Mode
B, B+B, B+M, M, 4B
Image gray scale
256 scale
Cine loop
≥500 Frame
Image storage
64 Frame
Scale Angle
Adjustable
Scan depth
40mm~240mm
Image flip
Up/down,left/right,black/white
Image Process
GAMA,Image Smoothen.THI,Histogram,Zoom
Focus
Focus Number,Focus position,Focus space
Measure
Distance,circumference,area,volume,heart,GAFW,EDD
Character display
Date,clock,name,PID,age,sex,hospital name,doctor
Output report
4 types
USB port
USB2.0
Power consumption
200VA
Size
1220mm×665mm×468mm
Packing list:
1×Main Unit
1×96 elements R60, 3.5MHz multi-frequency convex probe
1X96 elements L40, 7.5MHz multi-frequency Linear
Probe
1×Lilon battery
1×Power adapter(AC110V~240V,2A,50/60Hz)
1×Operation Manual
1X3D external Ultrasound image working station
****Optional *****
96elements R13, 6.5MHz multi-frequency Transvaginal Probe
96 elements R20, 5.0MHz multi-frequency Micro-convex probe
96 elements L40, 7.5MHz multi-frequency Rectal probe
Video thermal recorder (Original -- UP-897MD)
This product bears CE mark and the notified body is 0482
EC certificate No.: 6730GB410160520
Report No.: 6730FS06F
Statement:
FDA Disclaimer:
Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(The seller's name: Helen Lee,City:Beijing , State: Beijing, Country: China, Telephone number: 86-18796658874)
This item has been cleaned and treated according to the manufacturer's instructions.
About Warranty:
We guarantees new equipment other than accessories to be free from defects in workmanship and materials for a period of
24 months
from the date of shipment under normal use and service.
Return Policy:
1. If you have a defective item which you want to return, please contact us
within 30 days
from you receive the item.
2. We will refund the money to you when we get the return items. Or replace item for you.
GOOD NOETS : Above 200$ ,we normally send the item via express delivery,such as UPS ,DHL ,TNT ,EMS ,safe and fast,normally take 7-10 business days to worldwide.
W
e
commit ourself to do 100% customer satisfactione .Thanks very much.
1).The trade is cross-border and Airmail is the cheapest post way, so it take a long time for delivery.
2).We are not Responsible for your Customs duty .
3).We don't add taxes, VAT or other hidden charges.
We accept payment by
PayPal
. PayPal enables you to make payment quickly and securely online.
1: We only accept payment via PayPal.
2: Please pay the money within 5 days after the auction ended.
3: Please offer home or mobile phone when the item's Value exceed 100(USD,EUR,AUD,GBP)
We are the manufacturer and distributor of medical equipments for more than 10 years.If you are interested in the distribution work, please feel free to contact us.
If you have any question,please feel free to contact us
before you open case or leave us negative feedback
,we'll do our best to help you .W
e
commit ourself to do 100% customer satisfactione .
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States (Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664); and CE Approved, TUV of Europe (Cert.No. : G1 10 02 50972 013). You can consult with the FDA's Center for Devices and Radiological Health.